Atomic Structure
Every object and matter in this world can be broken down into smaller particles (molecules) that are invisible to our naked eye. The matter is the composition of atoms that form the material and metallic substances.
The Atom is a tiny particle that can be divided further into a million parts having properties such as heat, temperature and melting point etc.
Let’s take an example. If we take a copper clad and divide into tiny molecules, it loses the properties. The size of an atom is 1000th million parts of a centimeter.
Atoms are present in sugar crystals, copper, iron, and water etc.
What is an Atom?
It is an atomic particle of Matter (solid, liquid, gaseous and plasma) that contains a positive nucleus around which negative electrons revolve in a cyclic manner. The Nucleus is composed of protons and neutrons. Protons and neutrons are often called nucleons. The mass of an atom is almost the nucleus only.
Properties of Atom
- The atom is stable in nature.
- They are spherical in shape
- The mass of an atom is small compared to the nucleus
- Nucleus of atom made up of neutrons and protons
Relation between Atom, Nucleus, and Electron
The bonding between an atom and nucleus is like the relation between a mother and children playing around her. In this case, the mother is a nucleus and electrons are children.
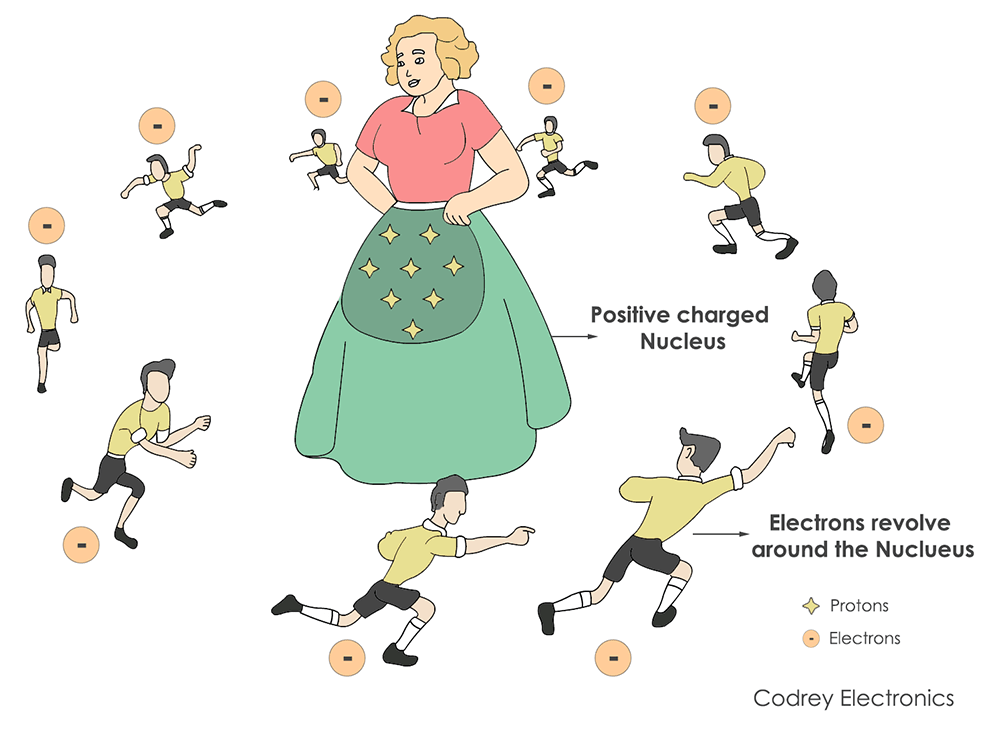
Electrons carry voltage and current in the form of an electric charge. An atom contains electrons and protons that revolve around the nucleus. The nucleus is the central piece of an atom controlling the electric charge outside the world.
The electrons are not attracted towards each other but they move towards the positive nucleus. The cross-section of the nucleus is much smaller than the atom and the electron is much smaller than the atom.
Practically, I can say there is nothing in the atom due to the smaller size of an electron.
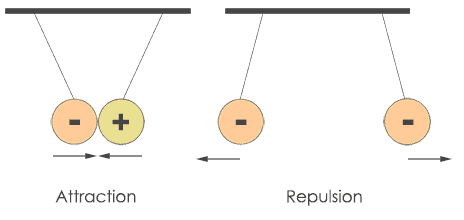
The nucleus exhibits different charges of electricity which are opposite in reality when compared to electron charge. By nature, the electron is negatively charged and the nucleus is positively charged.
According to Coulombs Law in electricity same charges repel and unlike charges attract each other, and this formula applies to the movement of electrons around the nucleus.
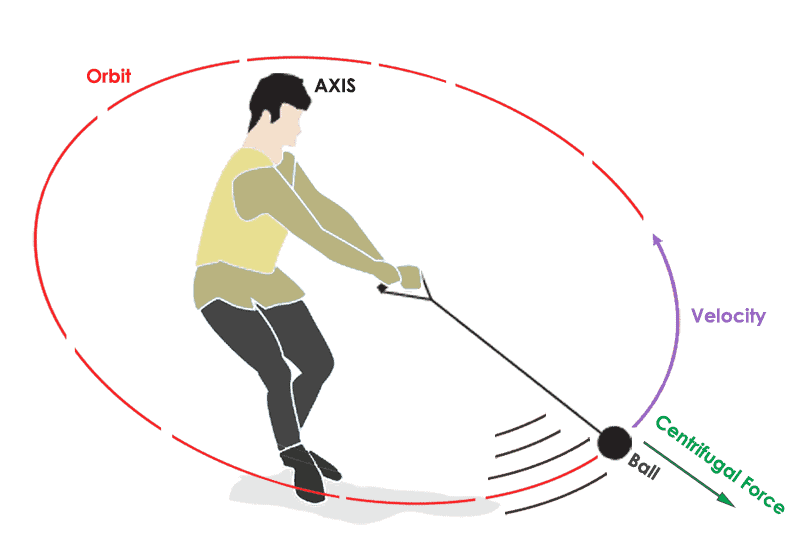
The fact you would not expect is that the nucleus will not attract the electrons due to the centrifugal force.
In the above figure, when the person is swinging the ball with high speed the centrifugal force acts between the person and the ball. This force makes the separation between the person and ball.
Likewise, the electrons (ball) whirl around the nucleus (person) at a high velocity, creating a centrifugal force keeping them away from the nucleus.
Electron in Atom
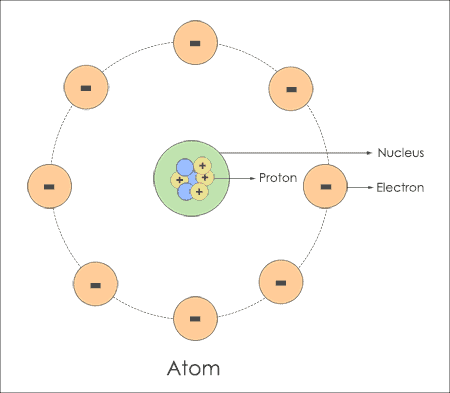
Electrons circulate close to the nucleus in different orbits and it differs with the type of material you use.
For example, Hydrogen atom has 1 electron and single nucleus and Gold has 79 electrons encircling behind the nucleus. The movement of electrons is not known as they roam through different tracks around the nucleus. It may choose circular orbit or elliptical path depending upon the type of chemical element and its properties.
As the charge of the nucleus is positive it neutralizes the electron making the atom neutral in nature. So, some of the electrons staying in the outer orbits have less attraction towards the nucleus. The nucleus situated outside the atom is called extra nucleus.
This attraction influences the electron to get isolated from the atom. The electrons leaving the atom (free electrons) drift inducing the electricity.
Now, the atom has a shortage of the electrons and becomes a positive ion.
Conclusion
Current and voltage are the main things for running an electronic circuit. But, behind them, quantum physics is involved. So, it is important to know the basic working and properties of electrons and atomic structure.